Key takeaways:
- Why is project management software critical in pharmaceuticals? Pharma projects are usually long-term, data-heavy, and highly regulated. Specialized software helps manage compliance, coordinate cross-functional teams, track risks, and build detailed audit trails.
- How does it improve efficiency and control? Wrike will replace spreadsheets and manual systems with automated workflows, real-time dashboards, and structured approvals. As a result, teams can reduce errors, close process gaps, and speed up reporting.
- What challenges does it help solve? From clinical trials to marketing launches, project management tools centralize data, streamline collaboration across departments, and adapt to complex lifecycles — ensuring projects stay compliant, efficient, and on track.
Pharmaceutical projects are specialized, highly regulated, and — in the case of projects like clinical trials — quite often long-term. But, despite this complexity, pharmaceutical teams often manage their projects using spreadsheets and databases rather than specialized project management software.
There are some very good reasons to make the switch to a centralized, automated platform to manage your next projects. When you work in research and development, manufacturing, pharmaceutical marketing, or even portfolio management, project management software can:
- Manage compliance workflows by tracking each of your projects through the same watertight stages
- Coordinate all the separate teams, like clinical, regulatory, and quality assurance, that have a role to play in your project
- Manage risk to your project, including your timeline, any regulatory requirements, and your vendor selection decisions
- Build a comprehensive project record, which makes it far easier to audit and report on your results, even when your project runs to thousands of separate data points
Put simply, pharmaceutical project management software helps your team work more efficiently without compromising on documentation or compliance.
Here, I’ll introduce you to Wrike, a scalable, secure, automated project management platform that’s built to handle the complexities of a pharmaceutical project — whether it’s a short-term product launch campaign or a project that spans years of research.
Later, I’ll show you how Wrike excels in various pharmaceutical use cases. But first, let’s run through the key features and the benefits of switching to a centralized project management tool.
4 reasons to manage pharmaceutical projects with Wrike
Wrike is a robust, flexible work management tool for cross-functional teams. Our platform is completely customizable and scalable, which makes it a fantastic option for pharmaceutical companies managing long project lifecycles, extensive portfolios, and the vast volume of data that comes with a complex project.
In my experience, teams in the healthcare or pharmaceutical industry tend to choose one of two methods to manage their projects:
- They use a system of shared spreadsheets: Pharma teams often work with spreadsheets because it’s a default way of recording data — and usually because they’re part of legacy systems. The problem is, this method sees them spend hours on manual data entry. Spreadsheets are open to errors, they make it tough to train new team members, and generating project reports can be an extremely complex challenge.
- They use a simple project management tool: When they move on from spreadsheets, many teams will implement a basic PM software solution (at least for some aspects of their manager’s work). But if they choose an entry-level platform, they struggle to get the right level of detail in their compliance workflows, they lack the features for cross-team collaboration, and they quickly hit a ceiling in terms of the number of tasks and projects they can manage.
Compared to these options, Wrike standardizes all your interconnected pharmaceutical project management systems and scales up as you need to. It can help pharmaceutical teams in a variety of ways, which we’ll cover in more detail below.
1. Handle complex, long-term projects with ease
The pharmaceutical industry is almost unique in terms of the length of the project lifecycle, the amount of data involved, and the rigid policies around retaining that data. In answer to all those challenges, Wrike sets itself apart.
Where pharmaceutical development projects can span 10+ years, Wrike is a robust platform that grows as your project does. For example, if your projects are based on drug discovery, Wrike’s tools take you from initial findings to research and development, through clinical trials, and then support you as you report on your results and bring a new product to market.
At every stage of your project, Wrike organizes your data and discussions in secure project folders. This helps you maintain a complete history of your project, and even the history of the individual tasks.
Best of all, you can call up any of this data, as needed, with a simple search. When it comes to auditing, reporting, or putting together a dossier for FDA approval, these features are invaluable.
Where pharmaceutical projects have critical deadlines, Wrike manages your project timelines proactively. For example, if your project planning has to accommodate a strict cutoff point to secure funding for the next phase, Wrike will visualize that deadline, monitor your project progress and time-tracking data, and alert you if your tasks are in danger of missing that goal. 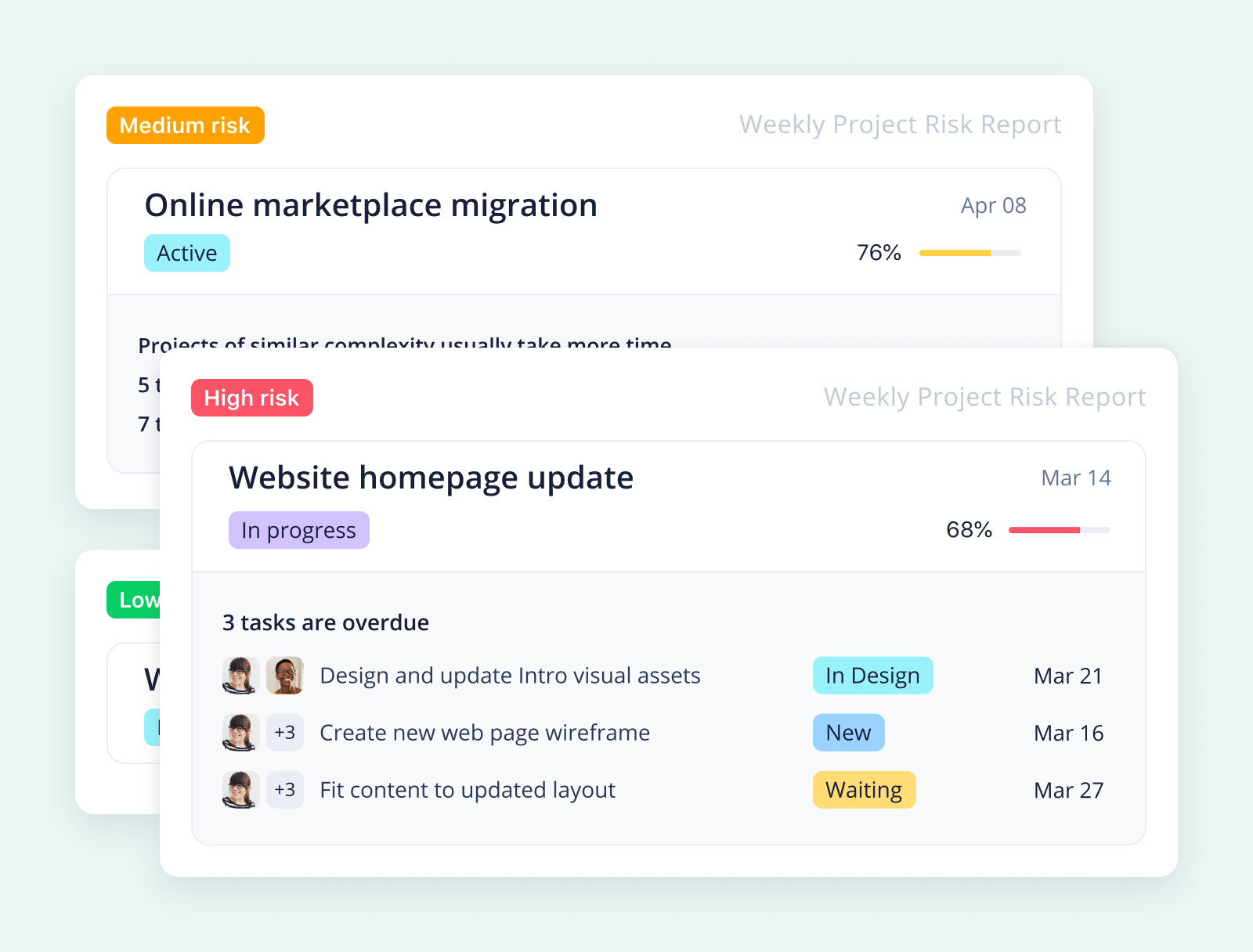
As well as centralizing your project overviews and discussions, Wrike helps you understand the complex dependencies within your project, and even forecast the ways that potential changes could ripple out across your timeline.
In a shared Gantt chart, for example, you can highlight dependent tasks and milestones with a simple drag and drop. Then, if there’s a change earlier in the project (for example, a delay in your supply chain), the dates of the subsequent tasks will adjust automatically.
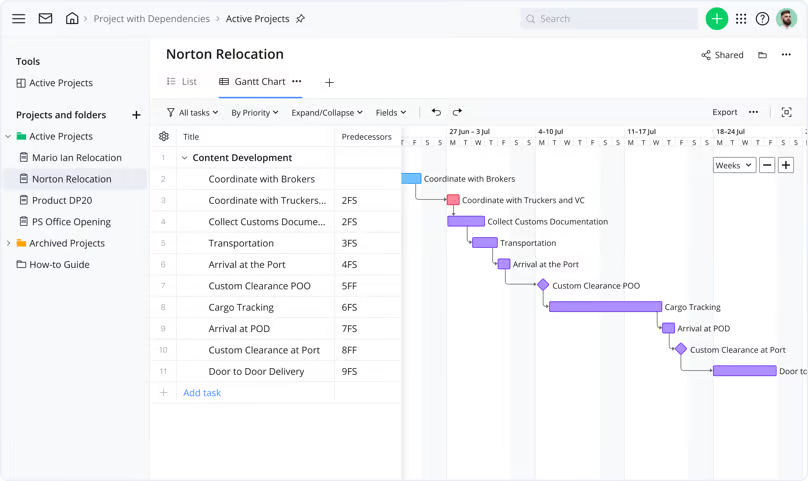
Tools like this help you view your project from every possible angle — from the workload for subteams like pharmacologists, toxicologists, trial managers, and statisticians to the project progress as a whole. And when all your datasets are transparent, searchable, and organized, you can make informed decisions about even the most complex pharma projects.
2. Close gaps in your project’s organizational structure
As well as handling the complexities of a long-term project, Wrike helps pharmaceutical companies manage the challenges of working with a sprawling, cross-functional team.
Without a centralized system for project and resource management, separate teams of scientists, statisticians, lawyers, and managers will likely use their own tools for project monitoring, file sharing, and communication.
This mix of separate tools creates massive gaps in your project management processes and compromises project tracking. In contrast, Wrike’s range of customizable project management tools means that every subteam can create a workspace that streamlines their contribution to the project.
For example, one of the main advantages of Wrike is that our communication tools move your discussions out of email and into a centralized workspace.
With Wrike, you can discuss your project tasks on your project task cards, with comments and @mentions that are saved securely so you can return to them later. This removes many of the barriers to cross-team collaboration and speeds up information sharing at every stage of your project. 
For example, as you create shared dashboards and workspaces, you can adjust permission levels to control the team members and job roles that can view those dashboards, or even create locked spaces. This helps you build a clear hierarchical structure within your project.
And, as your project progresses, Wrike’s system of workflows and project tracking generates an audit trail automatically. Whether your workflows are structured or unstructured, this means that you can return to review your work for compliance tracking even after a phase of your project is closed.
3. Adapt to the unique pharma sales process
The complexity of the sales process is another element that sets pharmaceutical projects apart from other industries. New drugs, therapies, supplements, and devices in the pharma industry need many layers of approval and involve complex decisions in terms of vendor selection.
Again, Wrike’s centralized systems help you track your conversations, assemble the documentation necessary for regulatory compliance, and align all the stakeholders in your project.
Consider a seemingly simple project like producing marketing materials to supply to healthcare centers ahead of a new product launch. With Wrike, you can build a custom approval workflow to ensure you meet all the conditions for marketing the product before you meet with potential partners. 
- Internal review from the scientific and drug development teams
- Approval from regulatory teams — including specialists from different markets, if necessary
- Legal review and approval
- Brand approval to review elements like the tone of the materials and the images selected
- Final approval from the project manager or client with ultimate authority
Wrike can also automate your approval workflow to reduce the time your materials spend in review. For example, when one stage is complete, Wrike can route the files to your project folders for your audit trail and notify the next person in the chain of approval. 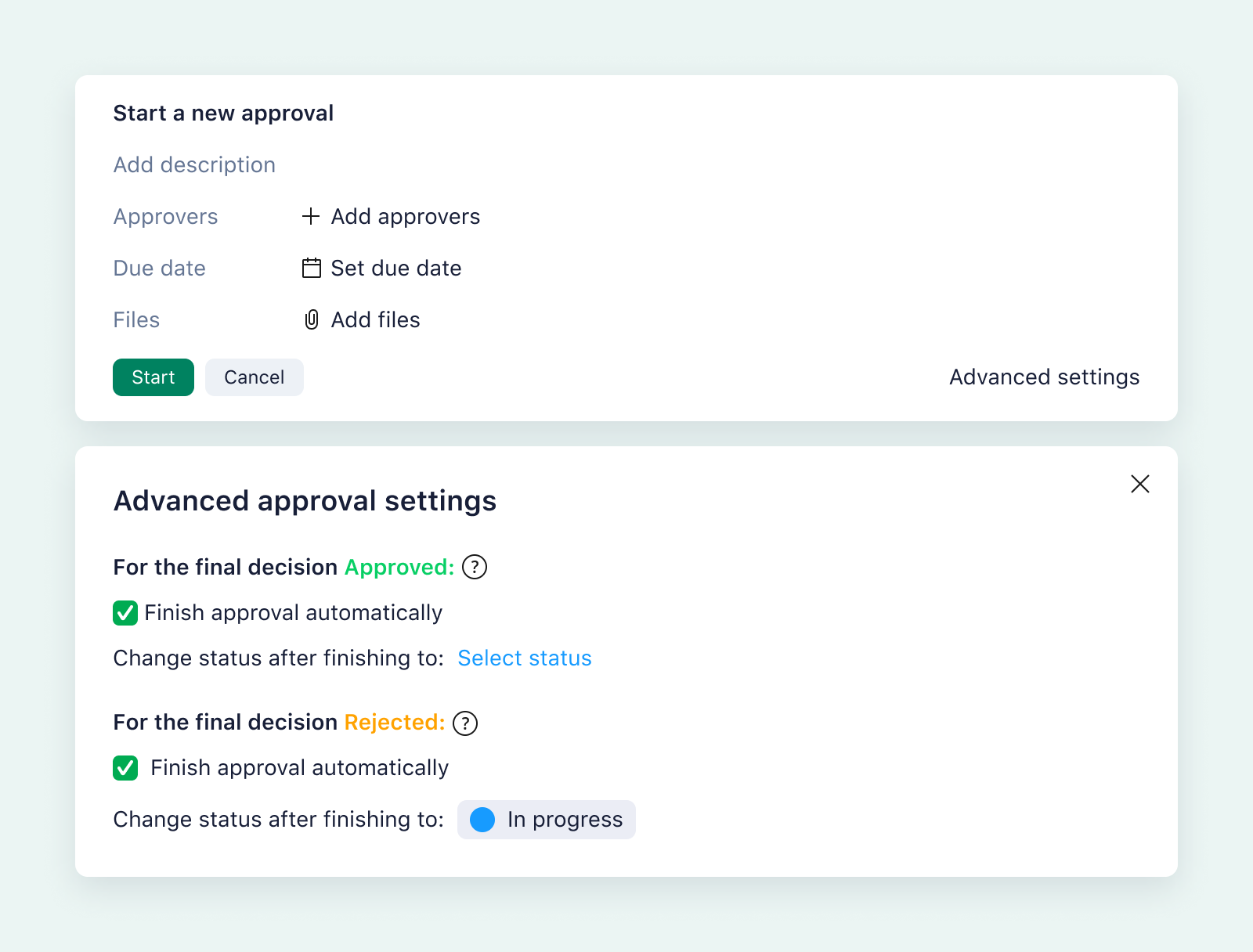
Combined, these features streamline the final stages of your project, dismantle bottlenecks and miscommunications in your review process, and significantly reduce your time-to-market.
4. Integrate the tools your projects rely on
When you work in Wrike, you can manage all your pharmaceutical workflows from end to end. This includes integrations with the other tools you use to complete your work.
Some of the most popular integrations for pharmaceutical teams include:
- Viva, a document management tool for routing and approvals
- Laboratory information management systems (LIMS)
- Clinical trial management systems (CTMS)
- Drug development platforms
- Research and development tools
With these integrations, you can make your existing processes more efficient without learning a range of new tools. Wrike means you can identify the best way of working, bring in the technology you’re already using, and standardize your approach every time your project cycle repeats. Wrike’s groundbreaking Work Intelligence AI will even identify new opportunities to integrate your tools and automate your workflows.
To find out more about how Wrike could integrate with your current stack — and other ways our project management software supports the unique needs of pharmaceutical teams — get in touch with our customer service team today.
How pharmaceutical companies are using Wrike
The pharmaceutical industry is broad and diverse. There’s no one-size-fits-all project management methodology to apply, and no out-of-the-box solution that guarantees project success.
During a recent Wrike Collaborate event, our customer panel session talked about the importance of adaptability in project management as the only way to maximize efficiency. This panel included Sherrie Besecker of Syneos Health, an end-to-end biopharmaceuticals company, and Kevin Thalacker of MilliporeSigma, a leading supplier for the life sciences industry.
Both experts spoke about how Wrike’s adaptability helped improve operational efficiency and project delivery in different areas of these large pharmaceutical companies. Their insights are included in these contrasting use cases.
Use case 1: Lab-based projects
Many of the pharmaceutical teams using Wrike have a research and development wing. This work is multi-disciplinary, data-heavy, and time sensitive — especially as projects are often funded by grants.
For lab-based projects, Wrike’s tools are invaluable for:
- Resource and equipment management. Wrike workspaces can now include asset scheduling and tracking features, which help you manage access to your lab and equipment and make the best possible use of your project time.
- Data management tools, including potential integrations with your LIMS, to record and organize your latest results and streamline the analysis of your results.
- Powerful reporting software to pull data from your records. You can use Wrike’s automatically generated, custom reports to support you in funding applications or present your initial findings without having to comb through spreadsheets or databases to compile them.
- Cross-functional collaboration tools to put your latest results and project plan in front of all the stakeholders bringing their expertise to the project or working on parallel experiments.
Speaking of the importance of collaboration in the pharma industry, Kevin Thalacker told us:
“When we started our journey with Wrike five years ago, we heard people say, ’I don’t want to deal with Microsoft Projects and update everyone’s project statuses.’ … I wanted to enable people. I wanted people to be collaborative. I wanted people to know what’s happening, and creating our single source of truth has been the key thing that I’m really proud of.”
With these shared tools and overviews, Wrike makes lab work flow more smoothly. You can plan and execute your work to make the most effective use of your resources, and you keep your data organized and auditable. Plus — to the extent that it’s possible in R&D — you accelerate your work by reducing manual tasks and speeding up communication.
Use case 2: Regulatory submissions
After new drugs, products, devices, or therapies have been developed, a regulatory submission is a project in itself. These projects can be particularly challenging because the process of documenting your findings can be unpredictable, but there’s often very little flexibility in the timeframe.
For example, imagine a team that has completed a Phase III clinical trial and is preparing to submit a new drug application (NDA) to the FDA. It can take as long as 18 months to present the results of the clinical trial and safety studies, solidify the manufacturing process, and develop the labelling required to bring the drug to market. Compiling the application involves several subteams and often thousands of pages of information and medical writing.
Organization is key, and Wrike gives you the tools you need to assemble the information and complete your application within the time available. These include:
- Project folders with version control for the documents you’re producing. When a document is cross-tagged within that folder, team members can work on the latest version without having to send duplicate files by email.
- Collaborative editing software, along with integrations with tools like Google Drive, to help your team create key texts together.
- Communication and commenting tools, which include automated notifications for the team members they concern, to help you record and action feedback on your early drafts.
- Document creation workflows, customized for your project, to help you monitor your progress and ensure that everything your team produces complies with the standardized formats required.
If your application receives feedback — like questions, or requests to supply additional documentation — you can also use Wrike to locate that information quickly and supply it before your application window closes.
Sherrie Besecker highlighted Wrike’s routing and review process as a massive time saver in this area:
“A big part of what we do is our routing and approval process, but what used to take us 8–10 steps using different systems now takes one.”
Use case 3: Pharmaceutical marketing
When you’re marketing new pharmaceutical products, you’ll face the same challenges as any marketing team. But you’ll also be up against more regulatory requirements, stricter approvals, and differing legal conditions in the separate markets you want to bring your product to. What’s more, the timing of these marketing campaigns is often crucial, as launches often need to align with clinical milestones, key conferences in the industry, or even seasonal markets.
Wrike is well-placed for marketing teams that operate within the pharmaceutical industry. Our tools include:
- End-to-end marketing workflows that take you from asset creation through to a multi-stage approval and compliance process
- Task tracking features like Kanban boards to streamline asset production and reduce bottlenecks
- Campaign management features for scheduling, tracking ROI, and managing campaign rollout across multiple channels
Together, these features help you design, prepare for, and launch a successful pharmaceutical marketing campaign to your ideal schedule.
Use case 4: Pharmaceutical portfolio management
When your company manages an entire pharmaceutical portfolio, you’ll face challenges maintaining an accurate overview of the ongoing work and its impact on the portfolio as a whole.
Imagine a nutraceutical company producing a range of supplements, probiotics, functional foods, and more. At any given time, they could be managing projects to:
- Develop new products
- Monitor sales and results from the products already on the shelves
- Update existing products to improve them and remain competitive
- Respond to regulatory changes
- Compile the documentation they need to move a product into a new market
Wrike is built for enterprise-level project portfolio management (PPM). All the features mentioned in this article scale to the portfolio level. This means Wrike can support your PMO with:
- Comprehensive portfolio dashboard visualizations to monitor upcoming, ongoing, and completed projects
- Real-time data to zoom in on individual projects, teams, or tasks and solve problems quickly
- Communication tools to check in with project managers and develop risk management or resource allocation strategies
- Robust reporting and data analysis tools to prioritize projects and inform the PMO’s decisions on the next projects to take on

When Sherrie speaks of her own experiences managing creative technology for Syneos Health, she highlights the scale of the project portfolio Wrike allows her to manage.
With tools like automated reporting, custom dashboards, and a standardized process of request forms, Syneos has used Wrike to:
- Issue 200+ tasks every day
- Complete 215,000 tasks in total
- Manage 15,000 successful projects

Wrike has become the lifeblood of our day-to-day operations — helping us achieve our important mission of bringing new therapies to patients more efficiently than ever.
Sherrie Besecker, Creative Technology Manager at Syneos Health
For robust, compliant project management, choose Wrike
Projects in the pharmaceutical industry are highly complex, but with Wrike, your project management software can be streamlined, intuitive, and connected to your entire global team.
When you use Wrike to plan your work, monitor your progress, and manage your project data, you:
- Protect the value of your project by avoiding waste and delays
- Reduce the risks to your project by maintaining strict compliance frameworks
- Document your long-term projects to preserve your findings and learnings
- Work flexibly and efficiently without compromising on control
Find out more about what Wrike can do for your pharmaceutical team today.